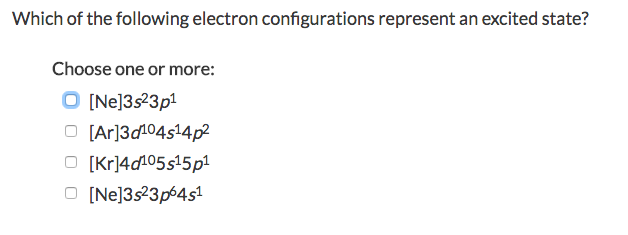
Which of the Following Electron Configurations Represent an Excited State
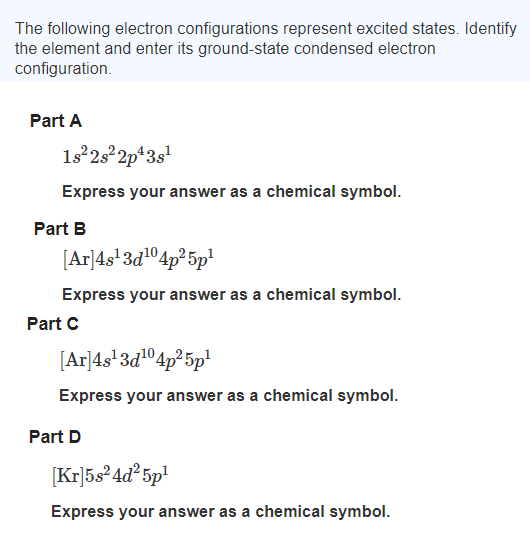
The following electron configurations represent excited states. The electron from a hydrogen atom drops from an excited state into the ground state.

Electronic Configuration Diagram Vs Energy For Carbon Atom In Its A Download Scientific Diagram
Which of the following electron configurations I-IV represents an excited state.
. It may represent an excited-state electron configuration of a Mg atom Which of the following may represent an excited-state electron configuration for a cobalt atom. Consider the following neutral electron configurations in which n has a constant value. Scroll down to electron shell properties click on that and see that the ground state for Lu is Xe5d1 6s2 and that makes Xe6s2 4f1 an excited state.
Xe6s24f1 2s2 1s22s22p63s23p63d2 Ar4s23d3 Kr5s14d5. Mg has atomic number of 12. An excited state of the element has the electron configuration 1s 2 2s 2 2p 5 3s 1.
Lol See Periodic Table See Hint Which of the following electron configurations represent ground states and which represent excited states. The ground state electronic configuration is 1 s 2 2 s 2 2 p 6 3 s 2. Identify the element and write its ground-state condensed electron configuration.
Up to 256 cash back Identify the following elements. Ar4s13d104p25p1 Express your answer as a chemical symbol. The following electron configurations represent excited states.
Which of the following electron configurations of neutral atoms represent excited states. Which of the following electron configurations represents an excited state of the indicated atom. Which of the following electron configurations represent an excited state.
An excited state of this element has the electron configuration Kr5s 2 4d 6 5p 2 6s 1. Identify the element and write its ground-state condensed electron configuration. Identify the element and write its ground-state condensed electron configuration.
Chemistry questions and answers. 1s2 2s2 2p6 3s2 3p2 4s1 Two elements that have the same ground-state valence shell configuration of ns2np2 are. If the element were to become excited the electron could.
Electron Configurations 4 items Drag and drop into the appropriate area below He2s 2p Ar3d1045-4p kr4d105525p Electronic State Ground State Excited State Ne3s 3p4s Drag and drop here. The ground-state electron configuration contains three unpaired 6p. Ill be glad to check the others for you.
1s2 2s2 2p6 3s2 3p2 3s1 bNe. For example if we look at the ground state electrons in the energetically lowest available orbital of oxygen the electron configuration is 1s2 2s2 2p4. Choose one or more.
The following electron configurations represent excited states. Which of the following electron configurations of neutral atoms represent excited states. A 1 s2 2 s2 2 p4 3 s1 b Ar 4 s1 3 d10 4 p2 5 p1 mathbfcmathrmKr 5 s2 4 d2 5 p1.
Write Rh ground-state condensed electron configuration. The following electron configurations represent excited states. Identify the element and write its ground-state condensed electron configuration.
Identify the element and write its ground-state condensed electron configuration. 1s2 2s2 2p6 cN. Which of the following electron configurations represents an excited state of the indicated atom.
1s2 2s2 2p6 3s2 3p2 4s1 eHe. Ls2 2s2 3p2 4p1. 1 s 2 2 s 2 2 p 6 3 s 1 3 p 1 represents an atom of magnesium in an excited state.
Thus it will have 12 electrons. Which of the following electron configurations of neutral atoms represent excited states. Write N ground-state condensed electron configuration.
Ar13d104s14p2 Kr14d105s5p1 Ne3s23p 4s1 2. The following electron configurations represent excited states. 1s2 2s2 2p3 dP.
Which electron configuration represents the electrons in an atom of ga in an excited state. 2 is He and that is the ground state. The ground-state electron configuration is Ne3s 2 3p 4 c.
A 1s2 2s2 3p2 4p1 b Ar 3d10 4s1 4p4 5s1 c Kr 4d65s25p1. Xe6s24f1 2s2 1s22s22p63s23p63d2 Ar4s23d3 Kr5s14d5. One electron is transferred from 3s to 3p level to form excited state.
Some instruments differentiate individual quanta of electromagnetic radiation based on their energies. So any electron configuration in which the last electron again the valence electron is in a higher energy orbital this element is said to be in an excited state. Group of answer choices aNa.
Write Se ground-state condensed electron configuration. Xe6s24f1 2s2 1s22s22p63s23p63d2 Ar4s23d3 Kr5s14d5 CHEM.
Solved The Following Electron Configurations Represent Chegg Com

Solved Which Of The Following Electron Configurations Chegg Com

Solved 1 Which Of The Following Electron Configurations Chegg Com
No comments for "Which of the Following Electron Configurations Represent an Excited State"
Post a Comment